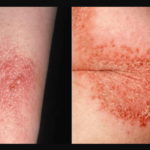
When hydrogen peroxide is applied as an antiseptic for small cuts, the skin area it is applied to becomes whiter. Why does this happen? Why does hydrogen peroxide turn skin white?
Hydrogen peroxide turns skin white because of a process called capillary embolism. This is a process in which blood does not flow to the capillaries, temporarily causing the color of the skin area whiter than the rest.
So, the skin area that comes in contact with hydrogen peroxide becomes whiter; albeit, temporarily. When the blood starts flowing back into the capillaries, the skin will return to its original color.
According to the Center for Disease Control and Prevention (CDCP), mild concentrations of hydrogen peroxide cause temporary irritation, and more concentrated solutions can cause severe burns.
The typical store-bought concentration of hydrogen peroxide used as an antiseptic or disinfectant for wounds is generally 3%.
There are various uses of hydrogen peroxide than being an antiseptic. Continue reading to learn more about why hydrogen peroxide turns skin white and how safe this antiseptic is.
Also, for an excellent cleanser, take a look at our top pick, the Erno Laszlo Brightening Dual Phase Peel:
Click here to see it on Amazon.
Why Does Hydrogen Peroxide Turn Skin White?
Hydrogen peroxide is commonly used in treating minor wounds (3% to 6% concentrations). It is a mild antiseptic that helps prevent infection because it stops the growth of bacteria.
Some recent studies, however, have shown that the disinfecting effect of hydrogen peroxide is minimal and that it doesn’t have a significant impact on the reduction of the growth of bacteria based on colony count.
Furthermore, it’s not appropriate for bigger wounds as these wounds need stronger antiseptics. [1] Nevertheless, many clinics and hospitals still use it to treat minor wounds.
Hydrogen peroxide is also a component of oxidizing agents in various household products (1.9% to 12%), such as bleach, hair whiteners, teeth whiteners, and nail whiteners. It could also be used to rinse the mouth of mucus from cold sores (3% to 6%).
Do not use hydrogen peroxide stronger than 10% concentrations as they are corrosive and can cause serious skin injuries. And, of course, never consume hydrogen peroxide, or you would be off to the hospital.
When applied to a small wound, it produces foam due to its oxidizing property when reacted with the enzyme catalase. Catalase is found in some bacteria, such as staphylococcus, in blood, and other substances.
When catalase comes in contact with hydrogen peroxide, a chemical reaction occurs, breaking it down to water (H2O) and oxygen (O2).
The foams are oxygen bubbles formed as an end product of the breakdown of hydrogen peroxide through the action of catalase.
Nonetheless, the foam fizzles out after a few minutes as oxygen is released into the air. Subsequently, the skin where hydrogen peroxide is applied becomes paler than the rest of the skin because of the capillary embolism process.
In capillary embolism or microembolism, blood does not flow temporarily into the capillaries in that area. So the skin becomes whiter than the rest of the skin.
Again, take note that this is only temporary because when the blood starts flowing back into the capillaries of your skin, your skin will go back to its original color. The whiter skin is also because of oxygen bubbles in the skin capillaries.
When the catalase reacts with hydrogen peroxide, oxygen bubbles are formed, diluting the blood and preventing smooth blood flow. This chemical reaction whitens the skin as blood is absent.
In simple terms, imagine your face drained of blood because of shock – you will look as pale as a ghost. That’s what happens with the skin that comes in contact with hydrogen peroxide.
With the release of oxygen, in the form of foams or oxygen bubbles, the breakdown of hydrogen peroxide in this phase is done.
Why Does White Foam Form When Putting Hydrogen Peroxide on Wounds?
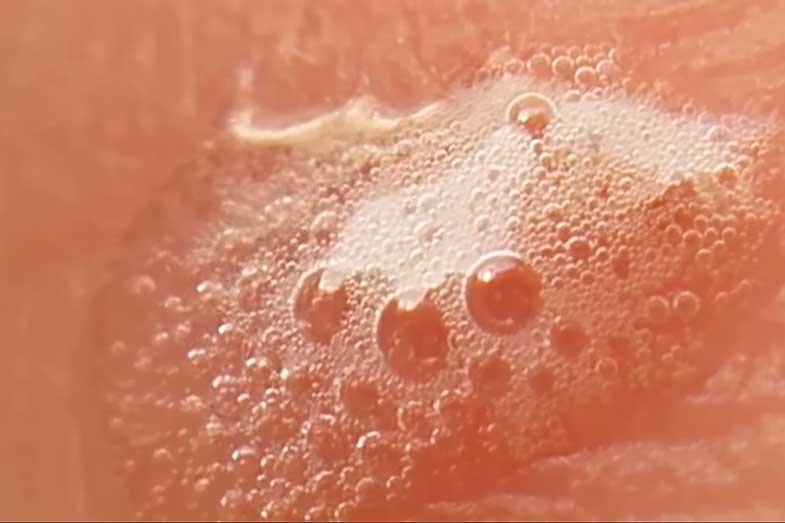
Why does hydrogen peroxide cause bubbles on wounds? It’s because hydrogen peroxide is an oxidizer (H2O2); it tends to produce white foams when applied on wounds that contain staphylococcus bacteria or blood cells.
This signifies that it’s interacting and killing bacteria through oxidation.
The majority of living organisms contain catalase, an enzyme that produces oxygen and water when it reacts with hydrogen peroxide. When blood, bacteria, and similar material interact with hydrogen peroxide, foam or bubbles are produced by the formation of oxygen and water.
When fizzling occurs, foams are produced as well. These are oxygen bubbles that are end products from the breakdown of hydrogen peroxide by the action of the enzyme catalase, which is present in bacteria, blood, and other microorganisms found in wounds.
This process indicates that the hydrogen peroxide is reacting with the bacteria and keeping them from infecting the wound.
So, the foam comes from the production of oxygen as an end product of the chemical reaction that takes place between catalase and hydrogen peroxide.
You can try a little experiment by applying a few drops of 3% hydrogen peroxide on your unbroken skin. Fizzing bubbles and foam will appear on the wound. It’s because your blood cells that contain catalase are not exposed primarily to hydrogen peroxide.
Unlike in wounds where blood cells are exposed directly, the blood cells’ catalase will react with hydrogen peroxide.
Also, wounds tend to attract bacteria, so together with the blood are bacteria that also contain the enzyme catalase. Anything containing catalase would react with hydrogen peroxide.
Josh Clark from Brain Stuf gives a good explanation of why hydrogen peroxide turns skin white when you add it to a wound:
Can Hydrogen Peroxide Turn Your Skin White Permanently?
Hydrogen peroxide cannot turn your skin white permanently. You should not assume that it’s a skin whitener, or you will kill your normal skin cells and injure yourself in the process too.
It’s dangerous to use a higher percentage of hydrogen peroxide as this can cause great damage to your skin and other organs in your body. You may want to know more about this from the studies presented below.
Again, the whitening of the skin is only temporary due to the physiological process (capillary embolism) that occurs upon the application of hydrogen peroxide. Using it daily with the idea of whitening your skin is not advisable.
Instead, for whitening and brightening the skin, you can use the following products:
The Sunday Riley Good Genes All-in-One Lactic Acid Treatment is Sunday Riley’s best seller. It brightens, exfoliates, and minimizes signs of aging.
Click here to see it on Amazon.
The Cellcosmet Precious Facial Mask brightens and illuminates your skin. It also helps even your skin tone, provides hydration plumps, calms, and soothes the skin.
Click here to see it on Amazon.
The Natura Bisse C+C Vitamin Body Cream is a creamy, rich emulsion with antioxidant properties. Like the Natura Bisse Diamond Glowing Mask, this Vitamin C body cream lightens the skin over time, making it brighter and more hydrated.
Click here to see it on Amazon.
Recommendations from Experts on the Use of Hydrogen Peroxide
There are some who drink hydrogen peroxide for some apparent health benefits. But do not drink it! According to the National Poison Center, swallowing 3% hydrogen peroxide is not too dangerous; however, high concentrations can cause skin irritation and burns.
Likewise, it can irritate the eyes. Drinking higher percentages of hydrogen peroxide can cause tissue burns. [2]
In another study conducted by a team of researchers led by Michael P. Lisanti, an expert in stem cell biology in Philadelphia, found out that hydrogen peroxide destroys the cells’ DNA and fuels aging, among other effects.
It could also act as a “fertilizer” for cancer metabolism and skin inflammation. [3]
Research published by the British Journal of Anaesthesia (BJA) and done by M. A. Akuji concluded that “hydrogen peroxide can do more harm than good.”
The study reported that 20 ml of 1.5% hydrogen peroxide is related to hemodynamic instability in adults, while even smaller amounts can cause cardiac arrest in children. [4]
These experts did not mention any whitening effects of hydrogen peroxide that are beneficial to your health. Rather, they have discovered that there are various harmful effects of the substance in your body.
Instead of taking chances, we would certainly use recommended skin whitening products that are FDA approved. Well, hydrogen peroxide is also approved for other uses, but not as a whitening product.
To learn more, see the article on HowChimp.com: Why Does Hydrogen Peroxide Turn Skin White?
Is Hydrogen Peroxide Safe for Skin?
Hydrogen peroxide is safe for skin at 3% to 6 % concentrations as an antiseptic. Concentrations higher than 10% can cause severe burns and irritation. Large volumes of even the lower concentrations are dangerous when kids ingest it.
Thus, don’t just leave your bottle where children can have access to it or leave it lying unguarded anywhere in your household.
Although it’s part of some foodstuffs, hydrogen peroxide – as is – should never be ingested purposefully. Even adults can have serious adverse reactions. You can go over the studies mentioned above. These are not opinions but results of scientific studies done in an empirical manner.
Conclusion – Why Does Hydrogen Peroxide Turn Skin White?
So, why does hydrogen peroxide turn skin white? This is because of capillary embolism or microembolism. In this process, hydrogen peroxide prevents blood flow into the capillaries of the skin.
This would temporarily cause paleness or whiteness of the skin because of the absence of blood. When blood starts flowing back after some time, the skin goes back to its normal color.
Simultaneously, foams or oxygen bubbles are formed as a result of the breakdown of hydrogen peroxide into water and oxygen by the action of the enzyme catalase.
Related reading:
How to Brighten Your Skin – 12 Tips
What Does Bleach Do to Your Skin?
How to Get Pale Skin? (Fast, Natural Tips)





![Neutral Skin Tone Defined [and Best Colors for Neutral Skin] neutral skin tone](https://skincaregeeks.com/wp-content/uploads/2021/05/neutral-skin-tone-150x150.png)